Pioneering Metalloprotease Targeted Therapeutics
PIPr-178 is poised to be the core of modern IBD therapy. Reach out to Heather Madsen at heather@hatterasvp.com to learn more.
Learn More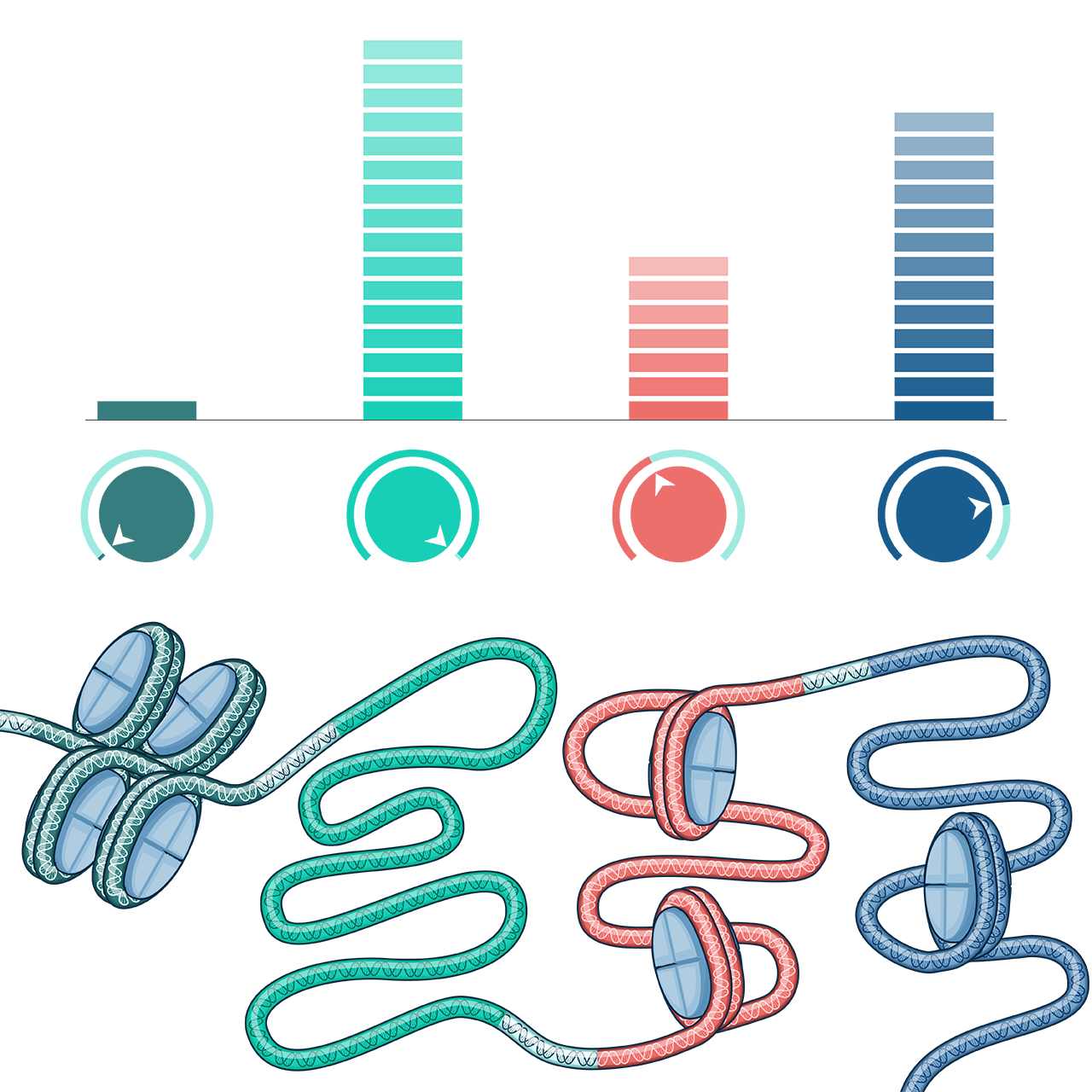







About PIPr-178
PIPr-178 is poised to be the core of modern IBD therapy, offering precision tissue targeting with novel metalloprotease inhibitors. Our innovative approach leads the way in the treatment of inflammatory diseases.

Precision tissue targeting with advanced metalloprotease inhibitors.

Lead asset, IND ready, with compelling safety and efficacy data.

11kg of GMP API ready for once-daily oral dosing development.

Integrated computational chemistry and ‘omics data mining.

Experienced team of drug developers and operators.

Raising $2.5M Seed to advance PIPr-178 towards clinical trials.
Technology Platform
Our technology platform integrates precision tissue targeting with advanced computational chemistry and ‘omics data mining to redefine therapeutic possibilities for inflammatory diseases.

Precision Tissue Targeting
Leveraging zinc-dependent metalloprotease inhibitors for targeted therapies.

Computational Chemistry
Defining targets and populations using advanced computational data analysis.

‘Omics Data Mining
Integrating data to identify precise therapeutic targets for better outcomes.

Advanced Analytics
Utilizing innovative analytics to enhance therapeutic efficacy and safety.

Development Expertise
A dedicated team driving innovation and advancing therapeutic solutions.

Clinical Focus
Focused on delivering groundbreaking therapies for inflammatory diseases.
Product Showcase
Discover PIPr-178, our flagship product designed to revolutionize IBD therapy with its precision-targeted approach and advanced metalloprotease technology.

IND Ready
PIPr-178 is IND ready with comprehensive preclinical efficacy and safety data.

Strong IP
Backed by strong intellectual property to ensure innovative development.

Manufacturing Ready
11kg of GMP API available for scalable production.

Dosing Convenience
Designed for once-daily oral dosing for maximum patient convenience.

Therapeutic Targeting
Precision targeting of inflammatory diseases with cutting-edge technology.

Clinical Focus
Focused on advancing PIPr-178 to clinical trials for patient benefit.
Our Team
Meet the experienced team of developers, operators, and innovators driving the success of PIPr Therapeutics.

Dr. Alex Johnson
Chief Executive Officer

Dr. Emily Carter
Chief Scientific Officer

John Smith
Head of Operations

Sarah Lee
Lead Data Scientist

Michael Brown
Clinical Research Manager

Jessica Taylor
Regulatory Affairs Specialist
Investment Opportunity
Join us in revolutionizing IBD therapy. PIPr Therapeutics is raising $2.5M Seed funding to advance our groundbreaking therapies to the clinic.


Pre-Clinical Data: Generating robust data to support PIPr-178 expansion.

Intellectual Property: Filing novel IP to strengthen our competitive edge.

Clinical Advancement: Advancing PIPr-178 towards clinical trials.

Expansion Hypothesis: Developing additional indications for PIPr-178.
Contact Us
Reach out to PIPr Therapeutics to learn more about our innovative solutions.
